STAR Series for Suzuki TXR
STAR Series for Suzuki TXR
 588 Series for Yamaha Ego 14″ and Suzuki Step 14″
588 Series for Yamaha Ego 14″ and Suzuki Step 14″
 399 SERIES For YAMAHA LAGENDA, YAMAHA LC 135.
399 SERIES For YAMAHA LAGENDA, YAMAHA LC 135.
FRONT SIZE : 160 X 17, REAR SIZE : 215 X 17 (White)
 399 SERIES For YAMAHA LAGENDA, YAMAHA LC 135.
399 SERIES For YAMAHA LAGENDA, YAMAHA LC 135.
FRONT SIZE : 160 X 17, REAR SIZE : 215 X 17 (Black)
 088 Series for Yamaha Ego 14″
088 Series for Yamaha Ego 14″
 519 Series for Honda Wave / Hurricane
519 Series for Honda Wave / Hurricane
 688 Series for YAMAHA LAGENDA Z, YAMAHA Y110, YAMAHA Y125Z, YAMAHA Y100, YAMAHA EGO / NOUVO (17″), HONDA WAVE 100 / WAVE 100 R / DREAM, HONDA WAVE 125, HONDA EX5, SUZUKI RGS, SUZUKI SMASH / RC, SUZUKI SMASH PRO / SHOGUN 125, MODENAS KRISS / KRISTAR (Drum Brake), MODENAS KRISS II / KRISTAR (Disc Brake)
688 Series for YAMAHA LAGENDA Z, YAMAHA Y110, YAMAHA Y125Z, YAMAHA Y100, YAMAHA EGO / NOUVO (17″), HONDA WAVE 100 / WAVE 100 R / DREAM, HONDA WAVE 125, HONDA EX5, SUZUKI RGS, SUZUKI SMASH / RC, SUZUKI SMASH PRO / SHOGUN 125, MODENAS KRISS / KRISTAR (Drum Brake), MODENAS KRISS II / KRISTAR (Disc Brake)
 811 Series for:
811 Series for:
Honda EX5 ( FRONT 140 X 17 / REAR 160 X 17 )
KRISS ( FRONT 140 X 17 / REAR 160 X 17 )
KRISS 2 ( FRONT 140 X 17 / REAR 160 X 17 )
Y110 ( FRONT 160 X 17 / REAR 185 X 17 )
Y125Z ( FRONT 160 X 17 / REAR 185 X 17 )
LAGENDA Z / LC 135 ( FRONT 160 X 17 / REAR 185 X 17 )
RXZ ( FRONT 185 X 17 / REAR 185 X 17 )
WAVE 125 II (MALAYSIA MODEL)
-( FRONT 160 X 17 / REAR 185 X 17 )
WAVE 125 (SINGAPORE MODEL)
– ( FRONT 160 X 17 / REAR 185 X 17 )
RG SPORT ( FRONT 160 X 17 / REAR 185 X 17 )
SMASH PRO ( FRONT 160 X 17 / REAR 185 X 17 )
SPECIAL MODEL : Y125Z RACE (FRONT 160 X 17 / REAR 250 X 17)
RXZ RACE (FRONT 185 X 17 / REAR 215 X 18)
LC 135 RACE (FRONT 185 X 17 / 215 X 17)
For more information, surf up to http://www.racingboy.com.my
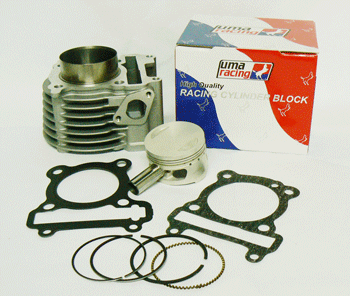 Uma Racing Engine Block for Yamaha Ego
Uma Racing Engine Block for Yamaha Ego