-
DO NOT take the battery for granted.
-
The only sure and safe way is to use a smart charger.
-
We’ve also put together a collection of OptiMate smart chargers you can consider.
We’ve previously published articles on how a motorcycle battery work and why they die (eventually). Let’s get down to battery maintenance in this instalment, plus a collection of OptiMate smart chargers.
Read: Motorcycle Battery: How it Works
Read: Motorcycle Battery: Why Does it Die?
Honestly, my biggest worry is always about a dead battery whenever I ride. It’s a big hassle and even downright scary when you’re stranded in the middle of somewhere in the middle of the night. Next to a cemetery.
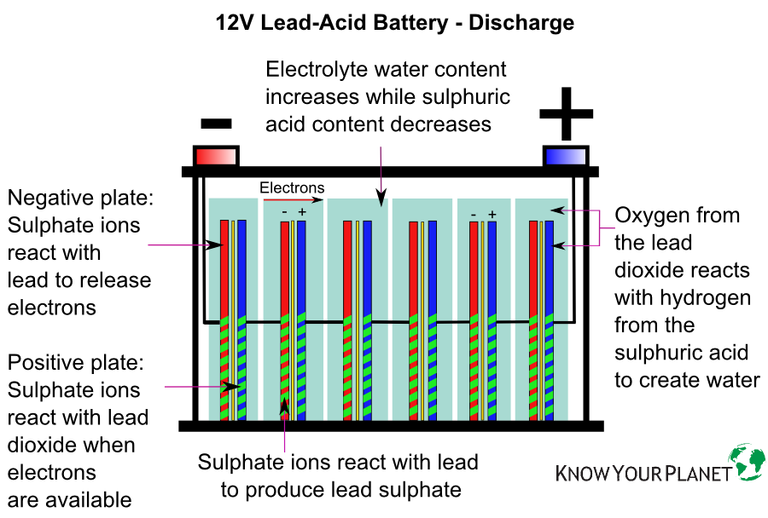
First and foremost, check the battery’s terminals. Make sure that the connectors don’t move about and that there are no deposits on them. Greenish copper sulfate may build up on the positive terminal, while a whitish sulfate can accumulate on the negative terminal (usually due to undercharging).
If there are, disconnect the battery – remove the negative connector first, followed by the positive side – and take it out of the bike. Then, use a toothbrush and scrub off the corroded material with some lemon juice or baking soda. Wash off with water and let dry.
CHARGING
Wish I could tell you otherwise but there’s no other better way to maintain your battery other than to use a smart charger. As we’ve mentioned before, the motorcycle’s charging system may not necessarily be the perfect charging system. Even if it is, you still need to maintain the battery at its optimum voltage once you stop the bike.
Charging a battery takes several steps with different voltages and amps, depending on its age and condition. This is where a smart charger does it all.
No, we’re not trying to hawk random stuff.
ENTER OPTIMATE
Among the chargers that I trust the most are OptiMate, made by the Belgian company TecMate. TecMate specializes only in battery chargers for all applications including powersports, cars, boats, commercial vehicles for over 20 years.
We know that there are many other chargers in the market, and many are amazingly cheap. But you need to ask yourself about why it’s so. Touch wood, using the wrong charger may not damage your bike’s battery or electrical systems but it may also not do any real good for the battery.
I’ve personally used a cheap charger which just kept pumping in the juice (overcharging) although the battery’s reached its optimum voltage (12.4 V). In the end, my battery warped out of shape. Am thankful that it didn’t explode!
An OptiMate, on the other hand, stops charging when the battery’s fully charged and then starts again as the volts start to drop. Just plug it in and leave it on.
The higher-end OptiMate 3, 4, 6, 7 and 8 have desulfation features. They do this by introducing electrical pulses to break the sulfur coating the cells and turn the electrolyte back to being acidic, rather than being more water. From there, these chargers will take further steps to charge and maintain the battery in the proper way. Heck, they even know what type of battery you’re using!
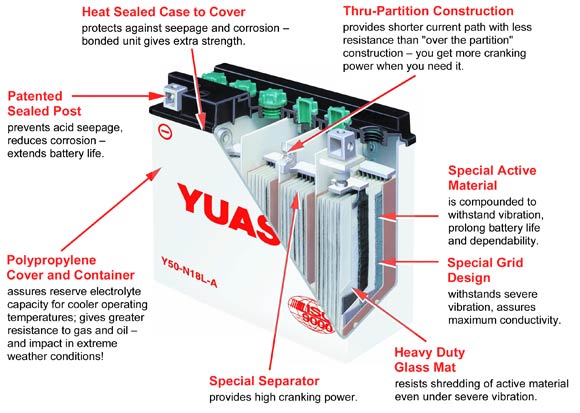
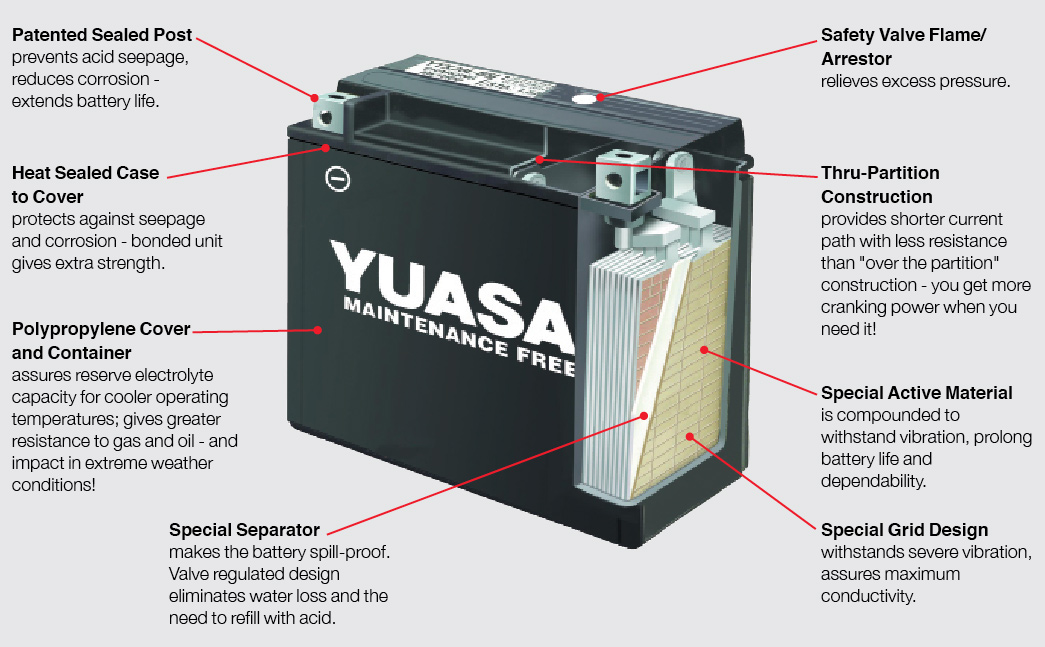
Included in each kit are two types of cables: The standard terminal clips and SAE-81 connector. The latter attaches permanently to the battery terminals, leaving the connector on the bike, and you don’t have to remove the seat or bodywork everytime you charge the battery. It also has a 15A fuse to protect against power surges.
Granted, a battery will die later in its life but at least the OptiMate chargers will test the condition of the battery and inform you. If the condition LED doesn’t turn green at all, it means it’s time for a new battery.
At least you don’t get stranded at night next to a cemetery…
NOTE
- DO NOT charge a LiFePO4 (lithium) battery with a standard lead-acid charger. The OptiMate 4S Lithium is designed specifically for lithium batteries.
- DO NOT use a standard charger if your bike uses CAN-BUS electrical and electronics wiring looms and connectors. Please use the OptiMate 4 CAN-BUS for that purpose.
WHERE TO BUY
You can take a look and buy an OptiMate smart charger in our BikesRepublic.com e-commerce site.
Please click >>> HERE <<< to visit the page.
Please click >>> HERE <<< for the OptiMate 2.
Please click >>> HERE <<< for the OptiMate 3 Dual Bank.
Please click >>> HERE <<< for the OptiMate 4 CAN-BUS.
Please click >>> HERE <<< for the OptiMate 4S Lithium.
Please click >>> HERE <<< for the OptiMate 6 Ampmatic.
Please click >>> HERE <<< for the OptiMate 7 Select.
Also available are USB chargers that plug into the 12 mm DIN power socket on certain bikes.
Please click >>> HERE <<< for the OptiMate O-105 Dual Output USB charger with 90-degree elbow.
Please click >>> HERE <<< for the OptiMate O-115 Dual Output USB charger.
OptiMate also offers a whole range of professional grade cables. You can always use them for your other motorcycles (for example).